Short paper on Super Spreader & Super Spreader Events
- Research Analysts, ETIO Public Health Consultants
- Jun 4, 2020
- 7 min read
Superspreaders and Super-spreading Events (SSEs):
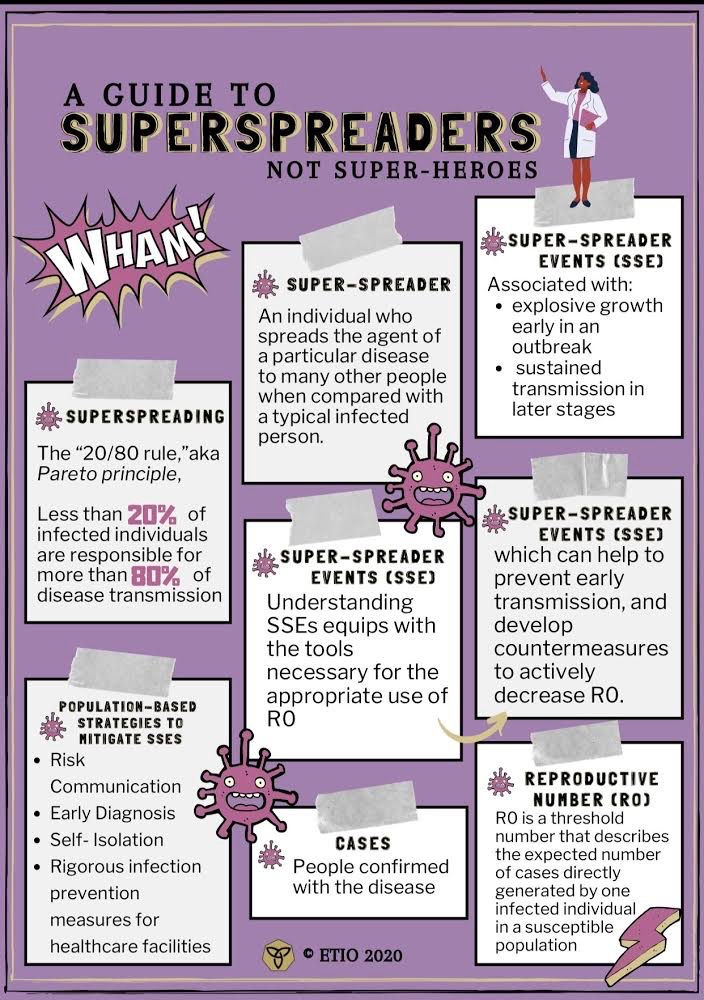
With continuous outbreaks of infectious diseases that continue to debilitate, perhaps “pause” the entire world, there is a need for improved control strategies to better understand host-pathogen interactions. It was previously thought that infected individuals within a population had equal chances of transmitting an infection to others (Stein, 2011). However, the “20/80 rule” was coined to define the observation that only a small percentage of individuals within a population contribute to transmission events. The “20/80 rule,” also known as the Pareto principle, proposes that less than 20% of infected individuals are responsible for more than 80% of disease transmission (Stein, 2011). This rule has offered insights into the features of pathogens and patterns of many infectious diseases, such as HIV, Measles, Ebola, Coronaviruses Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) (Stein, 2011).
Another epidemiological concept used to understand infectious diseases thus far is the concept of R0 (basic reproductive number). R0 is a threshold number that describes the expected number of cases (people confirmed with the disease) directly generated by one infected individual in a susceptible population (van den Driessche, 2017). Public health analysts aim to achieve an R0 that is less than 1 to indicate a decreased transmission rate. However, the concept of R0 is quite limited because although it does a good job of explaining population-wide infectiousness, it fails to show the accurate distribution of individual infectiousness. Hence, R0 should serve as only the first step in defining super spreading events and other steps must be taken to understand individual variation in infectiousness (Lloyd Smith et al., 2005). This revelation begs another insight into infectiousness known as super-spreading events (SSEs), typically associated with explosive growth early in an outbreak and sustained transmission in later stages (Frieden et al., 2020). SSEs highlight the limitation of R0, and this is evident with the COVID-19 pandemic. The R0 of COVID-19 was calculated to be between 2.79 (meaning 1 infected person will infect 2-3 people on average). However, there are instances where a person spreads the disease to a disproportionately larger number of people than the R0; this person is known as a super spreader.
A superspreader is an individual who spreads the agent of a particular disease to many other people when compared with a typical infected person (Stein, 2011). Understanding SSEs equips data analysts with the tools necessary for the appropriate use of R0, which can help to prevent early transmission, and develop countermeasures to actively decrease R0.
SSEs are known to be difficult to predict and therefore difficult to prevent since there are multifactorial shapers and drivers governing them (i.e co-infection with other pathogens, immune suppressions, delayed hospital admission, airflow dynamics, misdiagnosis, inter-hospital transfers, etc). Understanding the pathogen, host, environmental, and behavioural drivers of SSEs can inform strategies for SSE prevention and control and pandemic preparedness policies (Frieden et al., 2020; Stein, 2011).
Historical instances of SSEs:
Historically, the concept of SSEs has been documented with many infectious diseases. During the 2003 SARS outbreak, not all the patients with SARS were equally infectious, and the distribution of R0 gives a large Interquartile Range (IQR). According to the model framework by Chowell et al., (2004), the pooled R0 had an IQR 0.43-2.41, with a median of 1.10. In their search for the super spreading events for 2003 SARS epidemic in Hong Kong and Singapore, researchers (Li et al., 2004) indicated that more than 70% of infections were attributable to SSEs, with 71% of cases in Hongkong and 75% of cases in Singapore, respectively. By further analyzing the data made available from the University of Hong Kong, the researchers identified that delaying hospital admission (>4 days) could be another critical contributor for super spreading events, which highlights the importance of early diagnosis and self-isolation (Li et al., 2004). Similar findings are also featured in another analysis for the SARS outbreaks in Beijing (Shen et al., 2004), suggesting that the transmission of infection was not determined by the total number of symptomatic cases because not all the symptomatic individuals were able to infect others. By analyzing the data based on 77 patients, approximately 86% of patients did not infect others, 9% of patients infected three or fewer people, and the other 5% of patients infected more than eight contacts (Shen et al., 2004).
Similarly, the epidemiological data of the 2015 MERS-CoV outbreak in South Korea demonstrated significant heterogeneity in the transmission because 3% (n=5) of super-spreaders infected approximately 79% (n=149) of total MERS-CoV cases. Interestingly, one super-spreader alone contributed to 53% (n=79) of the total cases (Kim et al., 2018). In their investigation for clinical and epidemiological characteristics of spreaders of the MERS-CoV virus in Korea, researchers (Kang et al., 2017) found that 80% (n=4) of super-spreaders were male, and their clinical characteristics were slightly different compared to non-spreaders. For example, 60% of super-spreaders showed both dyspnea and myalgia, whereas only 19% of non-spreaders showed dyspnea and 42% of non-spreaders showed myalgia. Additionally, there was no significant difference in clinical parameters between super-spreaders and usual-spreaders (Kang et al., 2017). Nonetheless, given that the sample size for super-spreaders (n=5) and usual-spreaders (n=10) was relatively small, it is a challenge for health professionals to warrant a conclusive judgement on why a few patients infected a disproportionate number of their secondary contacts.
From a public health perspective, two crucial questions had remained as essential knowledge gaps in our understanding (Stein, 2011). Firstly, what makes individual patients become super-spreaders and how can health professionals find them on time? Additionally, there are other factors such as viral pathogenesis or environmental factors (i.e. crowding, airflow dynamics) that may change host-pathogen interactions. Therefore, the future prediction models need to consider the heterogeneity of each super-spreading event to warrant a more robust pandemic preparedness protocol.
Current Consequences of SSEs:
South Korea:
The first confirmed case of COVID-19 in South Korea was identified on January 20th, 2020. By late February, the virus had spread rapidly and the country had over 6200 confirmed cases of COVID-19 and over 40 confirmed deaths. The rapid spread of the SARS-CoV-2 virus is largely attributed to the actions of a single patient, Patient 31, who had attended church services a total of four times and had contact with 166 people. As a result, Patient 31 was responsible for causing more than 3900 secondary cases of COVID-19. Indeed, this case constitutes the phenomenon of “superspreading” because Patient 31 was responsible for spreading the virus to an unusually high number of people (Shim et al., 2020)
New Brunswick:
Recently, there have been at least eight confirmed cases of COVID-19 in the Campbelltown region, New Brunswick, linked to a family doctor, who did not self-isolate when he returned to work. Similar to SARS and MERS before it, the transmission of COVID-19 has been impacted by conspicuous SSEs, where there is a significant individual variation on transmissibility. In their search for the basic reproductive number of COVID-19, researchers estimated that the pooled R0 for COVID-19 was approximately 3.28 and with a median of 2.79 based on 33,738 confirmed cases (Liu et al., 2020). According to current understanding, the family doctor might infect more people than an average COVID-19 patient. Nonetheless, the current estimation of R0 for COVID-19 is potentially biased because the pathophysiology of COVID-19 is not fully understood by scientists. Therefore, the most feasible objective now is to find all the contacts with the doctor and take preventative measures to interrupt the further transmission of the disease.
Mitigation Strategies:
SSEs can be mitigated by numerous population-based strategies. The first population-based strategy involves risk communication. This entails continuing to stress the importance of physical distancing. The Center for Disease Control and Prevention (CDC) defines physical distancing as maintaining at least 6 feet of distance between other people, not gathering in groups and staying out of crowded places. This strategy aims to prevent the likelihood of encountering the SARS-CoV-2 virus, which can be spread via droplets, fomites and aerosol particles (Galbadage et al) and (van Doremalen et al., 2020). In addition, risk communication also entails continually stressing the importance of hand hygiene and avoiding face touching. This is because the virus can absorb through unprotected eyes and exposed mucous membranes.
Secondly, countries need to have the capacity to test, rapidly identify and diagnose patients. In fact, delay of diagnosis is the most common cause of SSEs (Frieden and Lee, 2020). Early identification and isolation of positive COVID-19 cases is therefore crucial to prevent SSEs and to prevent the overall spread of the virus.
Thirdly, healthcare facilities should implement rigorous infection prevention measures. Healthcare settings such as hospitals and care homes are very susceptible to SSEs (Frieden and Lee, 2020). For example, SARS-CoV-2 is present in stool which means that healthcare facilities need to ensure that toilets and other surfaces are free from contaminants.
Conclusion:
SSEs can contribute greatly to the spread of infectious diseases. Therefore, countries should implement numerous population-based measures to prevent superspreading.
References
Chowell, G., Castillo-Chavez, C., Fenimore, P. W., Kribs-Zaleta, C. M., Arriola, L., & Hyman, J. M. (2004). Model parameters and outbreak control for SARS. Emerging infectious diseases, 10(7), 1258–1263. https://doi.org/10.3201/eid1007.030647
Frieden, T. R., & Lee, C. T. (2020). Identifying and Interrupting Superspreading Events—Implications for Control of Severe Acute Respiratory Syndrome Coronavirus 2. Emerging Infectious Diseases, 26(6), 1059-1066. https://dx.doi.org/10.3201/eid2606.200495.
Galbadage, T., Peterson, B. M., & Gunasekera, R. S. (2020). Does COVID-19 Spread Through Droplets Alone?. Frontiers in public health, 8, 163. https://doi.org/10.3389/fpubh.2020.00163
Kang, C. K., Song, K. H., Choe, P. G., Park, W. B., Bang, J. H., Kim, E. S., Park, S. W., Kim, H. B., Kim, N. J., Cho, S. I., Lee, J. K., & Oh, M. D. (2017). Clinical and Epidemiologic Characteristics of Spreaders of Middle East Respiratory Syndrome Coronavirus during the 2015 Outbreak in Korea. Journal of Korean medical science, 32(5), 744–749. https://doi.org/10.3346/jkms.2017.32.5.744
Kim, Y., Ryu, H., & Lee, S. (2018). Agent-Based Modeling for Super-Spreading Events: A Case Study of MERS-CoV Transmission Dynamics in the Republic of Korea. International journal of environmental research and public health, 15(11), 2369. https://doi.org/10.3390/ijerph15112369
Li, Y., Yu, I. T., Xu, P., Lee, J. H., Wong, T. W., Ooi, P. L., & Sleigh, A. C. (2004). Predicting super spreading events during the 2003 severe acute respiratory syndrome epidemics in Hong Kong and Singapore. American journal of epidemiology, 160(8), 719–728. https://doi.org/10.1093/aje/kwh273
Liu, Y., Gayle, A., Wilder-Smith, A., & Rocklöv, J. (2020). The reproductive number of COVID-19 is higher compared to SARS coronavirus. Journal Of Travel Medicine, 27(2). doi: 10.1093/jtm/taaa021
Lloyd-Smith, J. O., Schreiber, S. J., Kopp, P. E., & Getz, W. M. (2005). Superspreading and the effect of individual variation on disease emergence. Nature, 438(7066), 355-359.
Shen, Z., Ning, F., Zhou, W., He, X., Lin, C., & Chin, D. et al. (2004). Superspreading SARS Events, Beijing, 2003. Emerging Infectious Diseases, 10(2), 256-260. doi: 10.3201/eid1002.030732
Shim, E., Tariq, A., Choi, W., Lee, Y., & Chowell, G. (2020). Transmission potential and severity of COVID-19 in South Korea. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases, 93, 339–344. https://doi.org/10.1016/j.ijid.2020.03.031
Stein, R. A. (2011). Super-spreaders in infectious diseases. International Journal of Infectious Diseases, 15(8), e510-e513.
van den Driessche P. (2017). Reproduction numbers of infectious disease models. Infectious Disease Modelling, 2(3), 288–303. https://doi.org/10.1016/j.idm.2017.06.002
van Doremalen, N., Bushmaker, T., Morris, D., Holbrook, M., Gamble, A., Williamson, B., … Munster, V. (2020). Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. The New England Journal of Medicine, 382(16), 1564–1567. https://doi.org/10.1056/NEJMc2004973








Kommentare